Pharma Contract Research Organization (CRO) Services Market to Cross USD 36.66 Billion in 2025, Expanding at a CAGR of 10.04%
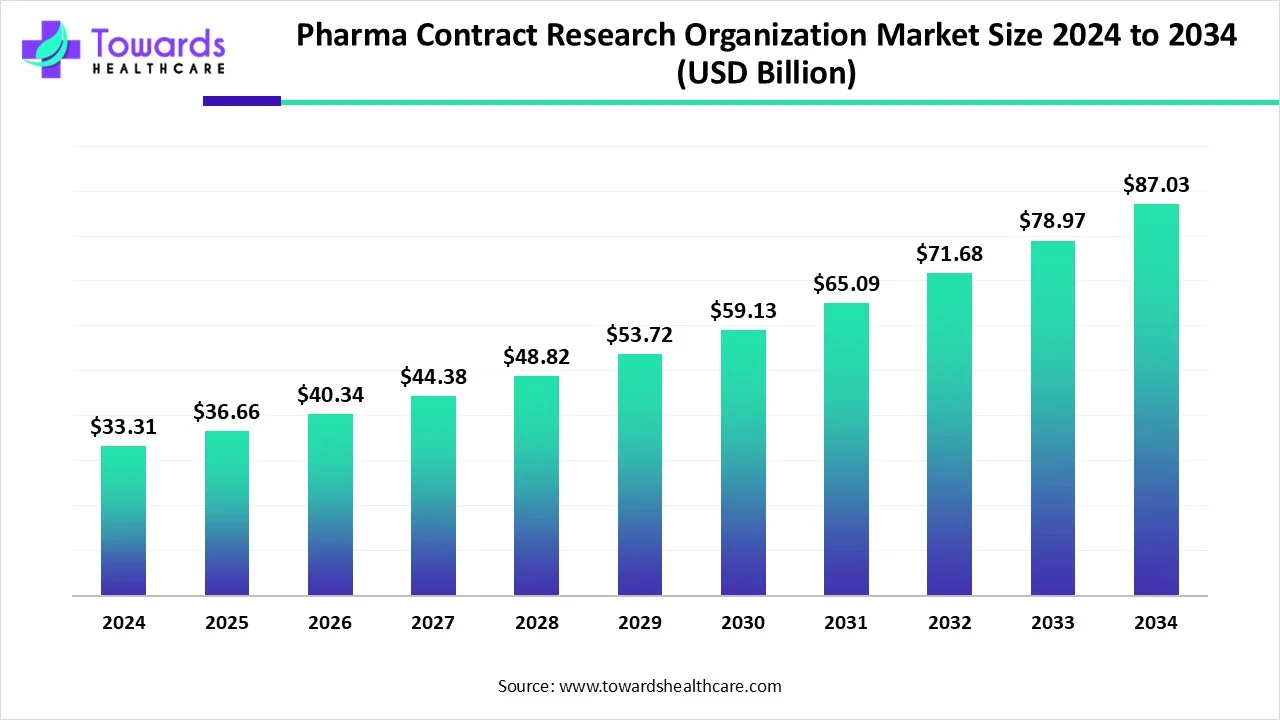
The global pharma contract research organization (CRO) services market size is calculated at USD 36.66 billion in 2025 and is expected to reach around USD 87.03 billion by 2034, growing at a CAGR of 10.04% for the forecasted period.
Ottawa, July 18, 2025 (GLOBE NEWSWIRE) -- The global pharma contract research organization (CRO) services market size was valued at USD 33.31 billion in 2024 and is predicted to hit around USD 87.03 billion by 2034, a study published by Towards Healthcare a sister firm of Precedence Research.
The growth of the market is driven by the growing factors like increased research and development spending, a growing number of clinical trials, and the rising prevalence of chronic diseases, which drives the growth of the market.
Get a quick preview of key market insights and trends shaping the Pharma CRO Services landscape: https://www.towardshealthcare.com/download-sample/5567
Key Takeaways
- North America dominated the global pharma contract research organization (CRO) services market in 2024.
- Asia-Pacific is anticipated to grow at the fastest rate in the market during the forecast period.
- By scale of operation, the discovery services segment held a dominant presence in the market in 2024.
- By scale of operation, the preclinical services segment is anticipated to grow at the fastest rate in the market during the studied years.
- By target therapeutic area, the oncological disorder segment held the largest share of the pharma contract research organization (CRO) services market in 2024.
- By target therapeutic area, the cardiovascular disorder segment is estimated to grow at a significant rate during the predicted timeframe.
Market Overview & Potential
A pharmaceutical Contract Research Organization (CRO) provides a range of research services to biotech, pharmaceutical, and medical device companies. These include preclinical studies, clinical trial management, regulatory support, and more, helping clients develop and get approval for new drugs and medical devices. CROs bring specialized expertise and resources that support the entire drug development process, from initial research to post-market monitoring. They offer benefits such as saving costs and time, access to expert knowledge, allowing clients to focus on their core activities, and increasing efficiency and speed, making them an attractive choice for consumers.
Access detailed data tables, segment analysis, and regional breakdowns in our comprehensive market databook: https://www.towardshealthcare.com/download-databook/5567
What is the Growth Potential Responsible for The Growth of The Pharma Contract Research Organization (CRO) Services Market?
The market growth is primarily fueled by the rising number of clinical trials, increased outsourcing of R&D activities, and the demand for cost-effective drug development solutions. Technological innovations, especially in AI and machine learning, along with the growing complexity of clinical trials, further drive the market. Additional growth factors include more clinical trials by pharma and biotech firms, cost efficiency, advanced technologies, complex trial designs, regulatory pressures, an expanding pipeline of new therapies, the rise of personalized medicine, and the broader scope of CRO services.
What Are the Growing Trends Associated with the Pharma Contract Research Organization (CRO) Services Market?
Outsourcing Of Clinical Trials
- The factors such as cost efficiency, specialised expertise, and advanced technology demand for outsourcing of clinical trial services.
Technological Advancement
- The integration of AI and ML for electronic data capture and electronic master files, and automating the processes, is a growing trend that drives the growth.
Focus On Personalized Medicine and Biologics
- The growing demand for personalized medicines and biologics catering to the needs of the patient and consumers drives the growth of the market.
Increased Research and Development Spending
- The growing pharmaceutical sector demand for research and development of new and innovative formulations, which demands CRO services, fuels market growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What Is the Growing Challenge in the Pharma Contract Research Organization (CRO) Services Market?
The key challenge that limits the growth of the market is the regulatory complexity, as different landscapes and regions demand significant expertise and resources, which is a challenge in the growth of the market. The high operational costs are also a challenge, like technological advancements, skilled labor, and regulatory compliance, con contribute to the high operational costs, which limit the growth of the market.
Regional Analysis
How Did North America Dominate the Pharma Contract Research Organization (CRO) Services Market in 2024?
North America dominated the global pharma market in 2024. The growth of the market is driven by technological advancements in the market to improve efficiency and speed of drug development through data analytics and AI fuels the growth of the market. The key players in the region also play a major role in the growth due to the offering and innovation in product development, which drives the growth like IQVIA, Laboratory Corporation of America Holding, Syneos Health, Parexel International Corporation, and ICON plc are some of the major players in the region. The growth is also seen as driven by research and development spending, focus on chronic diseases, and growing demand for outsourcing of research; these factors boost the growth of the market in the region.
Contract Research Organization (CRO) services in the U.S. are growing through increased outsourcing of clinical trials, rising biotech startups, and FDA support for faster approvals. Technological integration, decentralized trials, and access to diverse patient populations also drive expansion, making the U.S. a global hub for clinical research innovation.
Canada’s pharma contract research organization (CRO) services are expanding steadily through increasing biotech-pharma collaborations, government innovation funding, and adoption of virtual and AI-enabled clinical trials. Regulatory reliance on CROs for compliance, combined with growing oncology and rare-disease studies, is driving wider use across provinces.
What Made Asia Pacific Significantly Grow in The Pharma Contract Research Organization (CRO) Services Market In 2024?
Asia-Pacific is anticipated to grow at the fastest rate in the market during the forecast period. The growth of the market is driven by the increasing R&D spending, growing clinical trials, outsourcing trends, technological advancements, and cost effectiveness, which fuels the growth of the market in the region. The technological advancements and stringent regulatory standards attract companies that focus on the development of innovative drugs, which boosts the growth of the market. The expanding pharmaceutical companies, biotechnology firms, and medical devices companies in the region also promotes the growth of the market. The pricing and growing pool of skilled professionals in the region also contribute to the growth of the market.
CRO services in China are growing through supportive regulatory reforms, such as faster clinical trial approvals and acceptance of foreign trial data. The government encourages innovation, prompting CROs to expand capabilities across drug discovery, toxicology, and clinical operations. Collaborations between academic institutions and CROs are increasing, and the growing pool of trained clinical researchers is enhancing service quality. Additionally, many CROs are integrating digital tools and AI to streamline trial processes.
India's CRO growth is driven by a skilled, English-speaking workforce, a large and diverse patient population, and improvements in regulatory efficiency. Academic partnerships and government-backed innovation hubs foster research collaboration. CROs are adopting advanced technologies like remote monitoring, e-clinical platforms, and AI-based data analysis. Training programs enhance GCP compliance and clinical staff proficiency.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
Segmental Insights
By Scale Of Operation
The discovery services segment held a dominant presence in the market in 2024. Discovery services represent a foundational segment in the pharma contract research organization (CRO) services market, focusing on early-stage research to identify promising drug candidates. These services include target identification, hit discovery, lead optimization, and assay development, providing critical scientific insights before preclinical studies begin. By outsourcing discovery work, pharmaceutical companies can accelerate timelines, reduce costs, and access specialized expertise. The growing demand for innovative therapies and rapid pipeline expansion strongly supports the growth of discovery services within the CRO market.
The preclinical services segment is anticipated to grow at the fastest rate in the market during the studied period. Preclinical services are a crucial segment of the pharma contract research organization (CRO) services market, focusing on evaluating drug candidates’ safety and efficacy before human trials. These services include toxicology studies, pharmacokinetics, pharmacodynamics, and bioanalytical testing, providing essential data for regulatory submissions. By outsourcing preclinical work, pharmaceutical companies reduce development costs, access advanced technologies, and accelerate timelines. The increasing complexity of drug molecules and stricter regulatory standards drive demand for preclinical services, fueling the growth of the CRO market globally.
By Target Therapeutic Area
The oncological disorder segment held the largest share of the pharma contract research organization (CRO) services market in 2024. Oncological disorders represent a major target therapeutic area in the pharma market, driven by the urgent need for effective cancer treatments. CROs support pharmaceutical and biotech companies by providing specialized services across the drug development spectrum, including discovery, preclinical, and clinical studies focused on oncology. The complexity of cancer research, demand for targeted and personalized therapies, and rising global cancer incidence fuel this segment’s growth, making oncology one of the most heavily invested areas in CRO services worldwide.
The cardiovascular disorder segment is estimated to grow at a significant rate during the predicted timeframe. Cardiovascular disorders are a significant target therapeutic area in the pharma contract research organization (CRO) services market, driven by the high global prevalence of heart-related diseases. CROs support drug development for cardiovascular therapies through specialized discovery, preclinical, and clinical services, including safety assessments, efficacy studies, and biomarker analysis. The need for innovative treatments to address heart failure, hypertension, and related conditions fuels demand for CRO expertise. This strong focus supports market growth and advances the development of life-saving cardiovascular drugs worldwide.
Elevate your healthcare strategy with Towards Healthcare. Enhance efficiency and drive better outcomes schedule a call today: https://www.towardshealthcare.com/schedule-meeting
Recent Developments in the Pharma Contract Research Organization (CRO) Services Market
- In March 2025, LSK Global Pharma Services selected Oracle Argus to manage and expand its global pharmacovigilance operations, for the management of the databases of pharmaceutical companies, ensuring safety.
- In November 2024, Thermo Fisher Scientific launched a suite of expanded CRO and CDMO services under its brand. The company has introduced to the market its Accelerator™ Drug Development, which Thermo Fisher is marketing as “360°” CDMO and CRO drug development solutions.
Top Companies and Their Contributions to the Pharma Contract Research Organization (CRO) Services Market
| Company | Contributions & Offerings |
| IQVIA | A leader in data-driven CRO services, IQVIA offers clinical development, real-world evidence, and technology-enabled solutions globally. |
| Parexel International | Specializes in regulatory consulting, Phase I-IV clinical trials, and biotech partnerships, with a focus on patient-centric trials. |
| Medpace | Provides full-service clinical trial management, emphasizing therapeutic expertise and in-house services like labs and imaging. |
| Charles River Laboratories | Offers preclinical and early-phase clinical services, with expertise in drug discovery, safety assessment, and lab sciences. |
| CTI Clinical Trial & Consulting | Focuses on rare diseases and regenerative medicine, offering personalized CRO services from preclinical to commercialization. |
| WuXi AppTec | Delivers comprehensive R&D solutions, including lab testing, manufacturing, and clinical services, mainly serving pharma and biotech. |
| Veeda Clinical Research | India-based CRO offering cost-effective early and late-phase clinical trials, bioavailability and bioequivalence studies. |
| ICON plc | A global CRO with expertise across all phases, providing clinical development and commercialization support using advanced analytics. |
| LabCorp (Covance) | Integrates diagnostics and drug development, offering end-to-end clinical trial services through its Covance division. |
| Syneos Health | Blends clinical development with commercialization, offering biopharma integrated solutions and a strong site-network model. |
Top Companies in the Pharma Contract Research Organization (CRO) Services Market

- IQVIA
- Parexel International (MA) Corporation
- Medpace
- Charles River Laboratories
- CTI Clinical Trial & Consulting
- WuXi AppTec
- Veeda Clinical Research
- ICON plc
- Laboratory Corporation of America Holdings
- Syneos Health
Browse More Insights of Towards Healthcare:
-
Medical Device CRO Market:
The medical device contract research organization (CRO) market is valued at USD 8.49 billion in 2024, grows to USD 9.25 billion in 2025, and is expected to reach USD 19.9 billion by 2034, with a strong annual growth rate (CAGR) of 8.98%. -
Biopharmaceuticals CRO Market:
The biopharmaceuticals contract research organization market is expected to grow steadily from 2025 to 2034, potentially reaching hundreds of millions of dollars in revenue during the forecast period. -
Healthcare CRO Market:
The healthcare contract research organization (CRO) market is estimated at USD 53.87 billion in 2024, rises to USD 57.66 billion in 2025, and could reach USD 106.25 billion by 2034, growing at a CAGR of 7.04%. -
Preclinical CRO Market:
The preclinical contract research organization (CRO) market is expected to grow from USD 6.8 billion in 2025 to USD 14.34 billion by 2034, at a CAGR of 8.73%. -
Biologics CRO Market:
The biologics contract research organization market is valued at USD 31.15 billion in 2024, will grow to USD 35.22 billion in 2025, and is projected to reach USD 106.28 billion by 2034. -
Biotechnology & Pharmaceutical Services Market:
The biotechnology and pharmaceutical services market stands at USD 76.51 billion in 2024, increases to USD 80.7 billion in 2025, and is expected to reach USD 130.56 billion by 2034, growing at a CAGR of 5.48%. -
Pharmaceutical Spray Drying Market:
The pharmaceutical spray drying market is calculated at USD 2.37 billion in 2024, grows to USD 2.55 billion in 2025, and is projected to reach USD 4.93 billion by 2034, with a CAGR of 7.67%. -
Biopharmaceutical Third-Party Logistics Market:
The biopharmaceutical third-party logistics (3PL) market is estimated at USD 143.44 billion in 2024, grows to USD 152.93 billion in 2025, and could hit USD 276.24 billion by 2034. -
Cold Chain Pharmaceuticals Market:
The cold chain pharmaceuticals market is valued at USD 6.42 billion in 2024, increases to USD 6.67 billion in 2025, and is projected to reach USD 9.33 billion by 2034, with a CAGR of 3.83%. -
Radiopharmaceutical Market:
The radiopharmaceutical market is worth USD 6.8 billion in 2024, grows to USD 7.32 billion in 2025, and is expected to reach USD 14.11 billion by 2034, expanding at a CAGR of 7.57%.
Regions Covered
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Download the complete strategic report with deep analysis, forecast and competitive intelligence tailored for decision-makers: https://www.towardshealthcare.com/price/5567
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Gain access to the latest insights and statistics in the healthcare industry by subscribing to our Annual Membership. Stay updated on healthcare industry segmentation with detailed reports, market trends, and expert analysis tailored to your needs. Stay ahead of the curve with valuable resources and strategic recommendations. Join today to unlock a wealth of knowledge and opportunities in the dynamic world of healthcare: Get a Subscription
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

